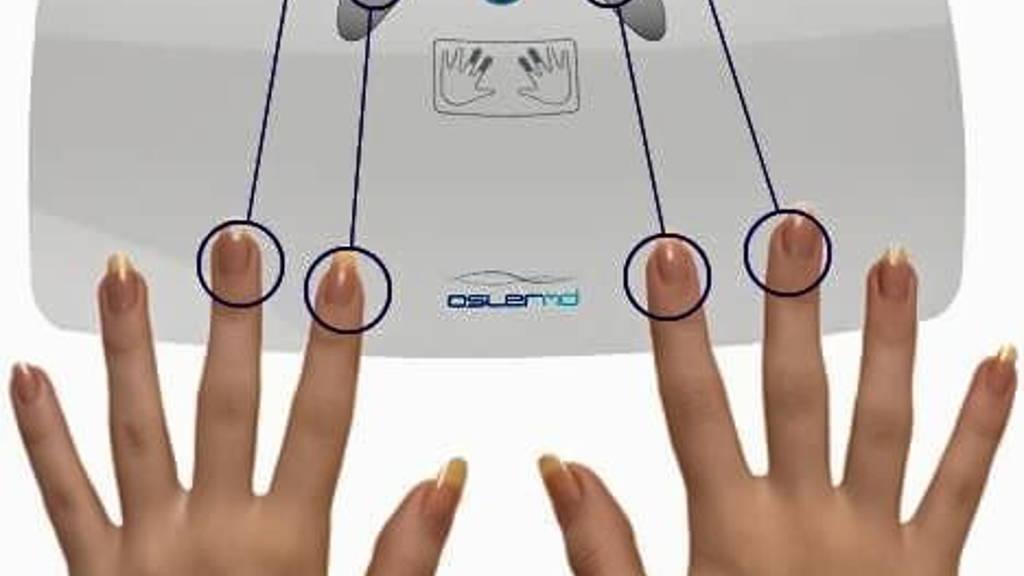
The device can be used in high traffic areas such as outpatient clinics, pharmacies, and schools, but also at home by post-op patients and others needing a vigilant eye to watch over their vitals, Medgadget writes. Six vital signs are recorded within thirty seconds.
The device doesn’t even have its own display. It relies on a tablet or smartphone to display the readings and to interact with additional devices. These devices – like a scale, a blood pressure cuff, or a glucometer, are each connected to theOslerMD system via Bluetooth.
The readings are compiled by an app (iOS and Android) that produces a comprehensive report based on various parameters measured. These reports can then be transferred to one’s medical team, as well as to family and any caretakers. All the actual calculations are performed on the cloud, outside the app, a feature that the company believes will make the regulatory path easier for them during software updates.
The company thinks about introducing cuffless blood pressure monitoring in the system. Because purely cuffless BP technology is yet to be clinically validated, OslerMD’s management team believes in ahybrid approach. This would involve a standard BP pressure cuff to make an initial measurement and then use “pulse pressure” readings from the fingers that would be adjusted based on the cuffed reading.
The device doesn’t even have its own display. It relies on a tablet or smartphone to display the readings and to interact with additional devices. These devices – like a scale, a blood pressure cuff, or a glucometer, are each connected to theOslerMD system via Bluetooth.
The readings are compiled by an app (iOS and Android) that produces a comprehensive report based on various parameters measured. These reports can then be transferred to one’s medical team, as well as to family and any caretakers. All the actual calculations are performed on the cloud, outside the app, a feature that the company believes will make the regulatory path easier for them during software updates.
Health Kiosk
According to OslerMD doctors’ offices can use the system during check-ins. The data can be routed to the default electronic medical records (EMR) used by the physician group. Since other devices can be tied into the OslerMD, the company eventually wants to deliver a ‘health kiosk’ based on its device that can be integrated into various health facilities.The company thinks about introducing cuffless blood pressure monitoring in the system. Because purely cuffless BP technology is yet to be clinically validated, OslerMD’s management team believes in ahybrid approach. This would involve a standard BP pressure cuff to make an initial measurement and then use “pulse pressure” readings from the fingers that would be adjusted based on the cuffed reading.