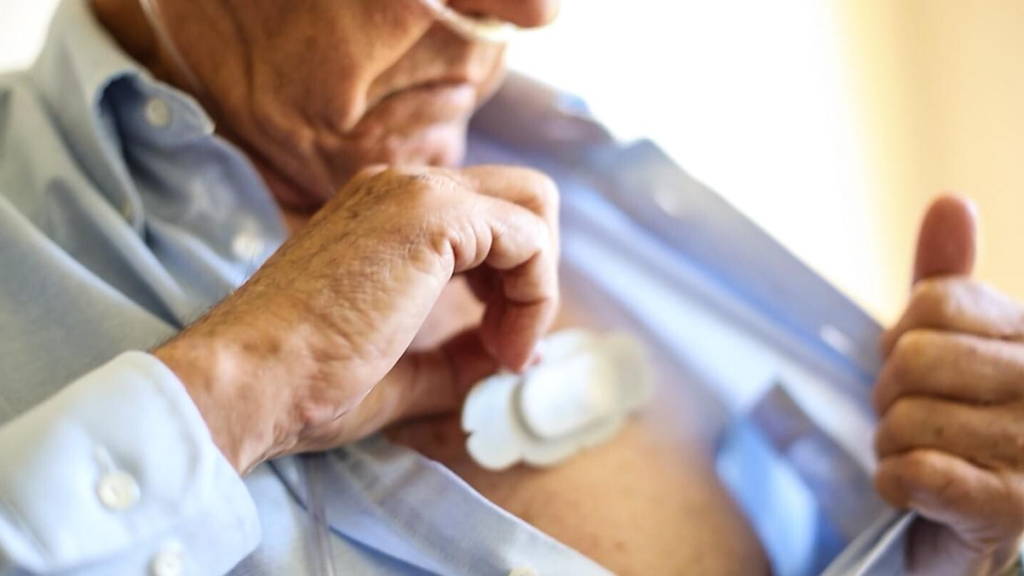
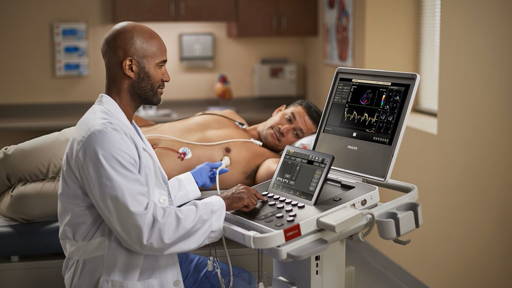
Up to one billion people globally are affected by chronic respiratory diseases—asthma, allergic rhinitis, and chronic obstructive pulmonary disease (COPD. While in the past, clinicians monitored patients’ vitals through regular checkups, new wearable technology now allows them to track this information remotely. ABI Research forecasts device connectivity will drive new chronic respiratory disease wearable form factors, with shipments to grow from three million shipments in 2017 to 12 million in 2022.
Recently ABI forecasted the entire patient monitoring wearable market, which includes remote and on-site devices, will grow from 8 million shipments in 2016 to 33 million in 2021. Device types are diverse and include staples like blood pressure monitors, continuous glucose monitors, and pulse oximeters, as well as newer devices like Fatigue Science’s fatigue monitoring wearable.
Non-invasive medical monitoring solutions developer Nonin Medical’s latest wrist-worn pulse oximeter—WristOx 2, Model 3150—measures patients’ oxygen saturation and pulse rates, transmitting the data using Bluetooth 2.0. Additionally, medical device manufacturer and startup Oxitone Medical recently released its Oxitone wrist pulse oximeter that continuously monitors blood oxygen levels and sends the data to an app via embedded Bluetooth Smart.
Beyond the hospital, remote patient monitoring wearables will provide healthcare professionals with continued access to their patients’ health, which would have otherwise been inaccessible once they left the confines of the hospital. On-site professional healthcare monitoring wearables allow medical professionals to work with a larger base of patients, as the devices continuously update doctors throughout the day on patients’ vitals and overall conditions without the need for physical check-ins.
Recently ABI forecasted the entire patient monitoring wearable market, which includes remote and on-site devices, will grow from 8 million shipments in 2016 to 33 million in 2021. Device types are diverse and include staples like blood pressure monitors, continuous glucose monitors, and pulse oximeters, as well as newer devices like Fatigue Science’s fatigue monitoring wearable.
U.S. spending highest
COPD, alone, costs U.S. healthcare systems $50 billion per year. ABI Research forecasts the U.S. will spend the most on chronic respiratory disease wearables, accounting for $6 million in 2022 of $21 million in global market revenues. Following the U.S., Europe and the Asia Pacific will also see high adoption rates. As the nascent market gains momentum, the devices will gradually shift from wireless finger-clips to wrist-worn devices.Wrist-worn wearables
“Wrist-worn chronic respiratory disease wearables are the ideal form factor, because they allow patients to carry out daily activities uninterrupted while collecting data continuously and in real time to note any subtle fluctuations in oxygen levels,” says Stephanie Lawrence, Research Analyst at ABI Research. “Finger-clip devices must be manually attached and activated throughout the day to provide one-off readings. Though they still provide more information than pulse oximeters in a clinical setting, finger-clip devices often miss subtle fluctuations that could indicate worsening conditions.”Non-invasive medical monitoring solutions developer Nonin Medical’s latest wrist-worn pulse oximeter—WristOx 2, Model 3150—measures patients’ oxygen saturation and pulse rates, transmitting the data using Bluetooth 2.0. Additionally, medical device manufacturer and startup Oxitone Medical recently released its Oxitone wrist pulse oximeter that continuously monitors blood oxygen levels and sends the data to an app via embedded Bluetooth Smart.
Overcoming challenges
Challenges vendors must overcome as the market develops, include meeting medical regulatory standards. The mandatory standards are critical, as they are clear proof that the device is an effective and accurate form of monitoring a patient’s condition. As obtaining medical regulation is a lengthy process, manufacturers must consider it in the early design stage as the global market develops.Beyond the hospital, remote patient monitoring wearables will provide healthcare professionals with continued access to their patients’ health, which would have otherwise been inaccessible once they left the confines of the hospital. On-site professional healthcare monitoring wearables allow medical professionals to work with a larger base of patients, as the devices continuously update doctors throughout the day on patients’ vitals and overall conditions without the need for physical check-ins.